From Mass. Bays Watershed Stewardship
Guide
Adapted from A Raindrop Journey, by Barbara Waters
ACTIVITY
Salt Water Wedge
|
Materials
Needed:
- Plastic
Box
- Cool
Water
- Cool Salty
Water
- White
Paper
- Paper
Cup
- Small
Stones
|
Grade Level: Grades
4-12
Time
Required: 1/2 -1 hour
Disciplines:
Science, Oceanography
Objectives:
- To be able to explain why
freshwater will stay at the surface while salt water will
travel up a river along the bottom in a wedge because of
density differences.
- To be able to describe the water
characteristics of an estuary, from salty ocean water, to
brackish, to fresh.
- To be able to identify estuary
areas in Massachusetts where freshwater meets the
sea.
|
Special Note:
To mix a seawater solution, add 35
grams of kosher salt (regular salt has additives) to 1 liter
of water or approximately 1.2 ounces (2 scant tablespoons) of
salt to 1 quart of water. To make a brackish mixture, halve the
amount of salt Tint the salt water with the food coloring.
Procedure: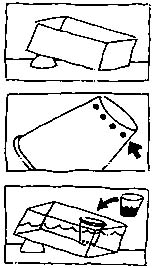
1. (Optional but recommended) Read the "Over
the Wedge" poster and "A Raindrop Journey." These publications are
available from the Mass. Bays Watershed Alliance (see below).
2. Place one end of the box on a small block
Place a piece of white paper under the box and fold the extra paper
up along the back to produce a white background.
3. Make several tiny holes in the bottom of the
cup. Weight the cup with small stones and place at the lower end of
the box.
4. Pour cool, fresh water into the box until it
reaches close to the top of the cup. Allow the water to settle.
5. Gently pour the cool, salty, tinted water
into the cup (do not overfill).
Q. Describe what
happens.
Q. Why?
|
INVESTIGATING
ESTUARIES
- Estuaries are an extremely valuable and productive
area. Research and report on why they are so valuable and
what sorts of resources are found there.
- Estuaries may be stratified in several layers due to
different salinity gradients. Try the experiment, first
using a brackish solution (with a red food dye)
and then a salt-water solution (with a blue food
dye) (make sure the water temperatures are all the same).
What do you observe?
- Make a visual representation of salinity differences
using two 16 ounce clear soda bottles. Fill one bottle
completely to the top with freshwater and the other with
salt water. Place some food dye in one bottle. With a
piece of cardboard over the mouth. insert one bottle over
the other. What happens? Try the experiment by changing
the solution that goes on top. What happens now? Which is
the halocline and which is the mixing process? The same
experiment can be done with solutions of varying
temperature to show thermoclines and temperature
currents.
- Take a field trip to an estuary. Test water samples
from various parts of the estuary with a commercial
hydrometer which measures salinity (the saltier the
water the more dense it is and the higher the
wading on the apparatus). Or build your own
homemade hydrometer. [Use a carrot or birthday candle
with 1". of copper wire twisted around the bottom as a
weight so that it just begins to sink if
placed in freshwater. Using two-liter bottles with
tops cut off for easy access or any two equal
vessels, fill to equal levels with samples of salt
and freshwater. Test your hydrometer -- either carrot
or candle - in each. Since salt water is denser
the hydrometer will float higher. Mark the level of
water. Test your hydrometer in brackish water and mark
the appropriate level.] Test the hydrometer with
various samples from an estuary.
- Follow directions for "The Great Hydrometer
Construction Contest!" in the April 1991 "Science
Teacher"
|
Hydrometer
Activity
Collect water to be tested for salinity and place in a
narrow container. Float the hydrometer in the container and
read the numbers where the narrow neck of the hydrometer
breaks the surface of the water [meniscus]. The
density [specific gravity] of the water determines
the buoyancy of the hydrometer. The more salt in the water
the more dense it is.
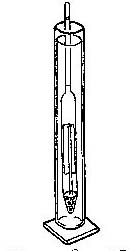
Fresh water has a salinity of 1.000.
Saltwater has a salinity of 1.20 to 1.30 depending on
temperature and dilution of saltwater with fresh. In an
estuary the saltwater from the open ocean is mixed with
fresh water coming from the land.
|
Stewardship Guide
developed by the Mass. Bays
Education Alliance for teachers, under sponsorship of Mass. Bays
Program and UMass. Extesion. Contact Faith Burbank to acquire a copy
of the Guide and A Raindrop Journey, at fburbank@gis.net or (781)
740-4913.

